2023.08.04
Adrenal Steroid Metabolites and Bone Status in Patients with Adrenal Incidentalomas and Hypercortisolism
Professor Yoshihiro Ogawa (Department of Medicine and Bioregulatory Science) has published their reaserch results.
For more information, please see below.
Adrenal Steroid Metabolites and Bone Status in Patients with Adrenal Incidentalomas and Hypercortisolism
Hiroshi Nakao, Maki Yokomoto-Umakoshi, Kohta Nakatani, Hironobu Umakoshi, Masatoshi Ogata, Tazuru Fukumoto, Hiroki Kaneko, Norifusa Iwahashi, Masamichi Fujita, Tatsuki Ogasawara, Yayoi Matsuda, Ryuichi Sakamoto, Yoshihiro Izumi, Takeshi Bamba, Yoshihiro Ogawa
Hiroshi Nakao, Maki Yokomoto-Umakoshi, Kohta Nakatani, Hironobu Umakoshi, Masatoshi Ogata, Tazuru Fukumoto, Hiroki Kaneko, Norifusa Iwahashi, Masamichi Fujita, Tatsuki Ogasawara, Yayoi Matsuda, Ryuichi Sakamoto, Yoshihiro Izumi, Takeshi Bamba, Yoshihiro Ogawa
For more information, please see below.
■Background
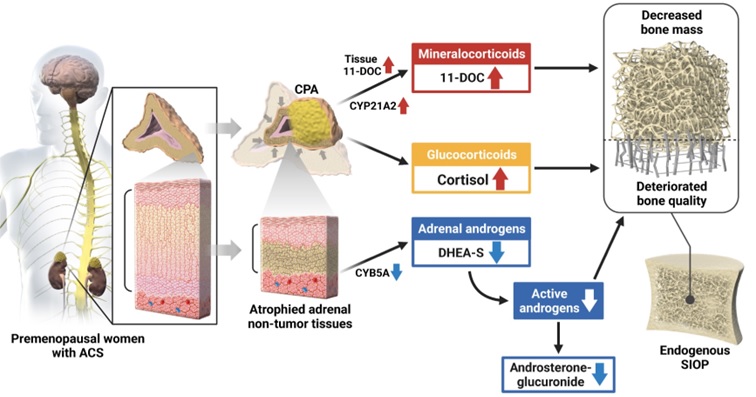
The adrenal cortex secretes a range of steroid hormones; mineralocorticoids, glucocorticoids, and adrenal androgens in a layer-specific manner. Evidence has suggested that the unbalanced production of adrenal steroid hormones contributes to the development of a variety of age-related disorders. Specifically, autonomous cortisol secretion (ACS), which results from cortisol-producing adenomas (CPA), is often associated with endogenous steroid-induced osteoporosis (SIOP), a major cause of secondary osteoporosis. However, the risk could not be explained by cortisol excess alone. Previous studies reported blood or urine steroid profiles in patients with ACS, but whether “adrenally-derived” steroid metabolites, affect bone status, has not been addressed.
■Summary of this study
Using liquid chromatography tandem mass spectrometry, we made simultaneous determination of steroid metabolites in both plasma and adrenal tissue samples from patients with ACS. Our data suggest that CPA co-secrete a mineralocorticoid metabolite; 11-deoxycorticosterone (11-DOC) and cortisol. In response to cortisol excess, there is marked atrophy of adrenal non-tumor tissues, with reduced production of adrenal androgens. In addition to cortisol excess, reduced adrenal androgens such as dehydroepiandrosterone sulfate (DHEA-S) are involved in deteriorated bone quality, while increased 11-DOC is involved in decreased bone mass, especially in premenopausal women. Collectively, this study demonstrates that the unbalanced production of adrenal steroid metabolites, which are derived from both adrenal tumor and non-tumor tissues, plays a critical role in the pathogenesis of endogenous SIOP.
■Clinical implications of this study
Through comprehensive steroid profiling, this study has uncovered the previously undescribed pathophysiological roles of adrenal steroid metabolites. Our findings indicate that these metabolites have differential effects on bone mass and quality. As such, we propose that osteoporosis occurs in patients with ACS as a result of combined actions of adrenal steroid metabolites. Our results have clinical implications, as they could aid in identifying individuals with ACS who are at a heightened risk of developing SIOP and inform the appropriate management strategies.